Introduction:
Autologous stem-cell transplantation (ASCT) is a standard of care for transplant-eligible patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL). ASCT-related costs and cost drivers remain unknown in Japan, thus restricting economic evaluation comparisons of newer innovative therapies with ASCT. Japan has universal health insurance coverage with a copayment system, which provides a nationwide snapshot of the current treatment landscape in Japan through health insurance database analysis. To capture continuous follow-up data of DLBCL patients who received stem-cell transplantation (SCT) in Japan, we conducted this study using a Japanese health insurance association (HIA) claims database.
Methods:
This is a retrospective observational study of patients with DLBCL who received SCT using the Medical Data Vision, Co. Ltd. (MDV; Tokyo, Japan) HIA claims database. The MDV HIA database contains inpatient, outpatient, and pharmacy claims from 149 health insurance societies in Japan. The database comprises approximately 7.84 million unique patient identifiers and covers about 3.0% of the Japanese population (as of August 2022). Patients who received treatment during the identification period from October 2012 to February 2022, inclusive, were selected using the DLBCL ICD-10 codes C83.3, C83.8, C85.1, and C85.2. Patients were required to have data for at least 6 months before and after the index date, which is the date of the first recorded ASCT procedure. The total cost included the costs of SCT-related lab tests, stem cell harvests, conditioning regimens during the lookback period, and all costs during the follow-up period including SCT-related hospitalization. Structural Equation Modelling (SEM) is ongoing to estimate the relationships of observed and latent variables having direct and indirect effects on pre-assumed causal relationships.
Results:
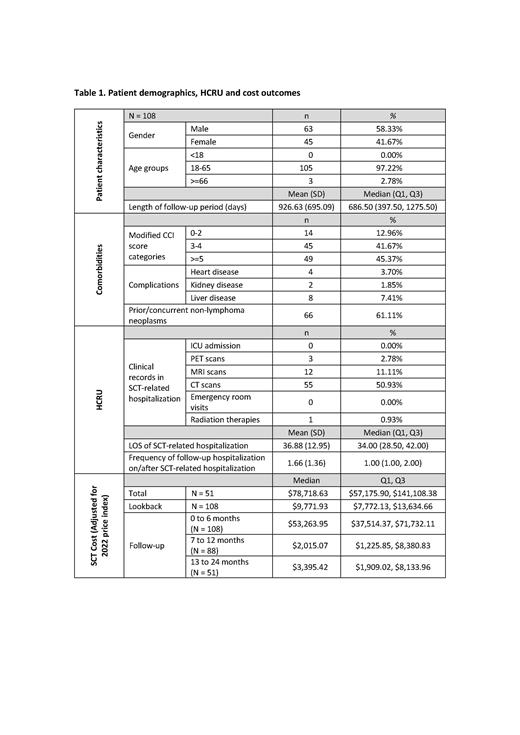
A total of 108 patients (3.8%) among all DLBCL patients who received SCT met the eligibility criteria, and all were ASCT patients. Of those patients, 41.67% were female and 97.22% were aged between 18 to 65 years old with a median age of 55. Subsequent therapy after the index date was received by 13.89% of the 108 patients, of which 2 (1.85%) patients received CAR-T cell therapy. Among the 108 ASCT patients, the median (Q1-Q3) length of SCT-related hospitalization was 34 days (28.50-42.00 days). During SCT-related hospitalization, CT scan was the most common test (22, 50.93%) among all ASCT patients, followed by MRI (12, 11.11%) and PET (3, 2.78%); and no patients were admitted to the ICU or emergency room during SCT-related hospitalization. There was 1 (0.93%) patient who received radiation therapy during SCT-related hospitalization. The median (Q1, Q3) of the total cost for ASCT patients during the lookback and follow-up period (index date inclusive) was $78,718.63 ($57,175.90, $141,108.38).
Conclusions:
This is the first study to examine the total cost and healthcare resource use of ASCT among patients with DLBCL in the Japan nationwide setting with a follow-up period of up to 24 months. While it is not directly comparable to our study, the median of the total cost for ASCT with a 6-month follow-up period in the US, as reported by Cui et al. (2022), was more than $86,408. The data presented in this study will be a benchmark when considering new innovative therapies for patients with DLBCL. Identifying the cost drivers of ASCT-related costs for DLBCL patients may provide insights for optimizing clinical practice for patients with DLBCL undergoing ASCT and for delivering better care for patients.
Disclosures
Tsutsué:Gilead Sciences: Current Employment. Makita:AstraZeneca: Honoraria; BMS: Honoraria; Gilead: Honoraria; Takeda: Honoraria; Genmab: Honoraria; Chugai: Honoraria; CSL Behring: Honoraria; Daiichi-Sankyo: Honoraria; Eisai: Honoraria; Novartis: Honoraria; SymBio: Honoraria. Asou:Gilead Sciences: Current Employment. Wada:IQVIA Solution Japan: Current Employment. Lee:IQVIA Solution Japan: Current Employment. Ainiwaer:IQVIA Solution Japan: Current Employment. Idehara:IQVIA Solution Japan: Current Employment. Aoyagi:IQVIA Solution Japan: Current Employment. Kim:IQVIA Solution Japan: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal